Motion Restoring Surgery adds over 200 days of Recovery
According to IBM Watson Health data, complications after initial orthopedic surgery, particularly joint contracture (M24.5x) caused by excessive scar tissue, can add over $10,000 to treatment costs and extend recovery by more than 200 days. Even when additional surgery isn’t performed, joint contracture significantly drives up healthcare utilization and delays outcomes.
ERMI Stretch Assist Machines provide a nonsurgical, home-based solution that helps patients regain mobility without the risks associated with surgery. When used for just two months, over 90% of patients with joint contractures avoid motion restoring surgery entirely. There have been no reported complications associated with ERMI’s devices, reinforcing their safety and efficacy. Early identification and treatment with ERMI technology not only prevent surgical escalation but also accelerate recovery timelines.
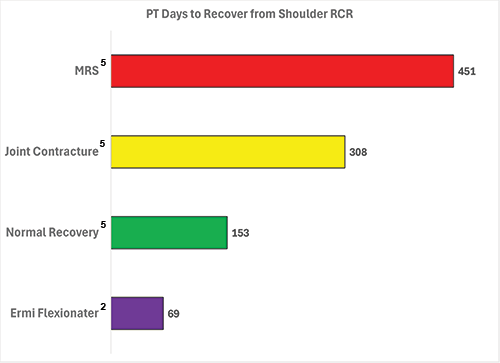
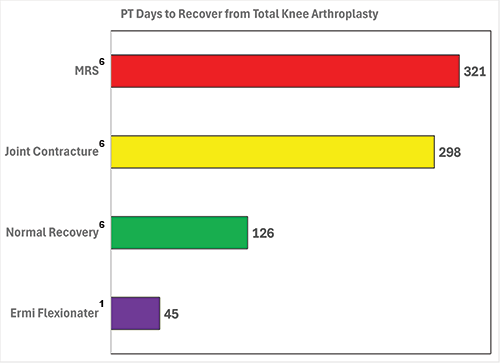
MRS = Motion Restoring Surgery. Joint Contracture = Joint Contracture M24.5x and/or Adhesive Capsulitis M75.0x
Clinically Proven, Non-Operative Success
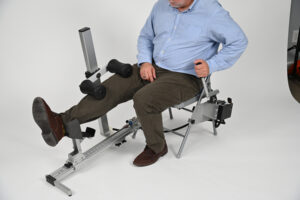
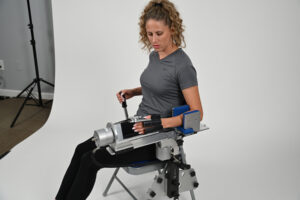
ERMI’s patented motion therapy devices, such as the Flexionater and Extensionater, are designed to restore joint range of motion effectively and efficiently. Clinical data support their success in helping patients regain mobility and avoid additional medical interventions. In many cases, patients using the Shoulder Flexionater have recovered motion equivalent to or exceeding post-surgical results, without the need for an operating room.
Avoid the Extended Downtime of Surgery
Motion-restoring surgery often results in prolonged recovery timelines, adding weeks or sometimes months of functional downtime, rehabilitation, and lost productivity. ERMI devices are designed to help patients recover faster, at home, and on their schedule, significantly reducing time away from work and daily activities. This streamlined recovery results in minimized disruption for patients and more efficient resource utilization for payers.
Home-Based Therapy That Puts Patients First
ERMI therapy devices enable patients to take an active role in their recovery from the comfort and safety of their own homes. This approach reduces the need for frequent in-person visits, alleviates the burden on physical therapy networks, and enables flexible, intensive rehabilitation that adapts to individual progress.
Value-Driven Clinical Integration
ERMI collaborates directly with physicians, physical therapists, and case managers to seamlessly integrate device usage into treatment pathways, resulting in consistent, measurable improvements in mobility while managing overall treatment costs.
Independent studies and clinical data have shown that patients using ERMI devices can avoid costly surgeries and reduce the number of in-clinic physical therapy sessions required. This reduction in healthcare utilization, combined with improved recovery outcomes, delivers significant long-term savings for payers. In many cases, ERMI’s approach helps shorten disability duration and accelerates return-to-work timelines.
Ideal for Diverse Populations
Our devices are currently covered and successfully utilized in Workers’ Compensation and Veterans’ Affairs populations, as well as in most cases, Commercial Insurance and Medicare populations. We focus on helping patients return to work and regular activity sooner, supporting better outcomes for both patients and the organizations that serve them.
Billing Practices
ERMI works closely with insurance carriers, third-party administrators, attorneys, healthcare providers, and patients to ensure accurate and efficient billing. Our team supports the reimbursement process by providing clear documentation and proactive communication, helping reduce administrative delays.
There is ongoing confusion in the classification of HCPCS billing codes for durable medical equipment, particularly when physical therapy procedural language is used to describe devices. This can result in different technologies, such as portable splints and stationary mechanical therapy devices, being grouped under the same code despite having distinctly different operating mechanisms and functions. These differences lead to different outcomes.
ERMI is actively engaged with the appropriate regulatory and coding bodies to support accurate classification of our devices. Our goal is to ensure that billing reflects the therapeutic value and proper use of our equipment while maintaining compliance across all payer systems.
Compliance and Regulatory Information
ERMI is fully committed to upholding the highest standards of regulatory compliance and patient safety. All ERMI Stretch Assist Devices are registered with the U.S. Food and Drug Administration (FDA) as Class I medical devices and are manufactured under applicable FDA regulations and Good Manufacturing Practices (GMP). Our devices are intended for home use and are prescribed by licensed healthcare providers.
ERMI maintains strict internal quality control protocols and regularly audits our operations to ensure adherence to federal, state, and payer-specific requirements. Our commitment to compliance encompasses patient data privacy and security, with all systems fully aligned with the Health Insurance Portability and Accountability Act (HIPAA).
Additionally, ERMI collaborates with physicians, case managers, and insurers to ensure accurate documentation, thorough utilization review, and adherence to evidence-based treatment plans.
Partner with ERMI
To learn how ERMI can support your mission of cost-effective, high-quality care, contact us:
Phone: (877) 503-0505
Email: info@ermi-motion.com
Address: 441 Armour Place NE, Atlanta, GA 30324
Standard of Care 



for severe motion loss
Lower Your Costs
This is custom heading element
90% Effective with Zero Risk
This is custom heading element
Lower Risk of Overuse
Strict compliance and use protocols ensure that only patients who need our solution receive it, and our Ermi professionals monitor patient progress to avoid overuse.
Sources:
* Papotto BA, Mills T. Treatment of severe flexion deficits following total knee arthroplasty: a randomized clinical trial. Orthopedic Nursing. 2012 Jan-Feb; 31(1):29-34.
** Stephenson JJ et al. Knee-attributable medical costs and risk of re-surgery among patients utilizing non-surgical treatment options for knee arthrofibrosis in a managed care population. CRMO. 2010; 26(5):1109-1118.
Our program is backed by scientific research and proven with patients to work. Browse through our materials to learn more about solving end range of motion issues.
Our Programs
All products are FDA Approved
CONTACT US
Get in Touch
If you have a question, concern, or issue, please fill out the short form and we will have an Ermi representative get in touch with you.
Atlanta, GA 30324